The US Food and Drug Administration recently approved Azedra (iobenguane I 131) injection for intravenous use for the treatment of adults and adolescents aged 12 and older with rare tumours of the adrenal gland (pheochromocytoma or paraganglioma) that can’t be surgically removed (unresectable), have spread beyond the original tumour site, and require systemic anticancer therapy.
This is the first FDA-approved drug for this use.
‘Many patients with these ultra-rare cancers can be treated with surgery or local therapies, but there are no effective systemic treatments for patients who experience tumour-related symptoms, such as high blood pressure,’ explained Richard Pazdur, MD, Director of the FDA’s Oncology Centre of Excellence, and Acting Director of the Office of Haematology and Oncology Products in the FDA’s Centre for Drug Evaluation and Research.
‘Patients will now have an approved therapy that has been shown to decrease the need for blood pressure medication and reduce tumour size in some patients.’
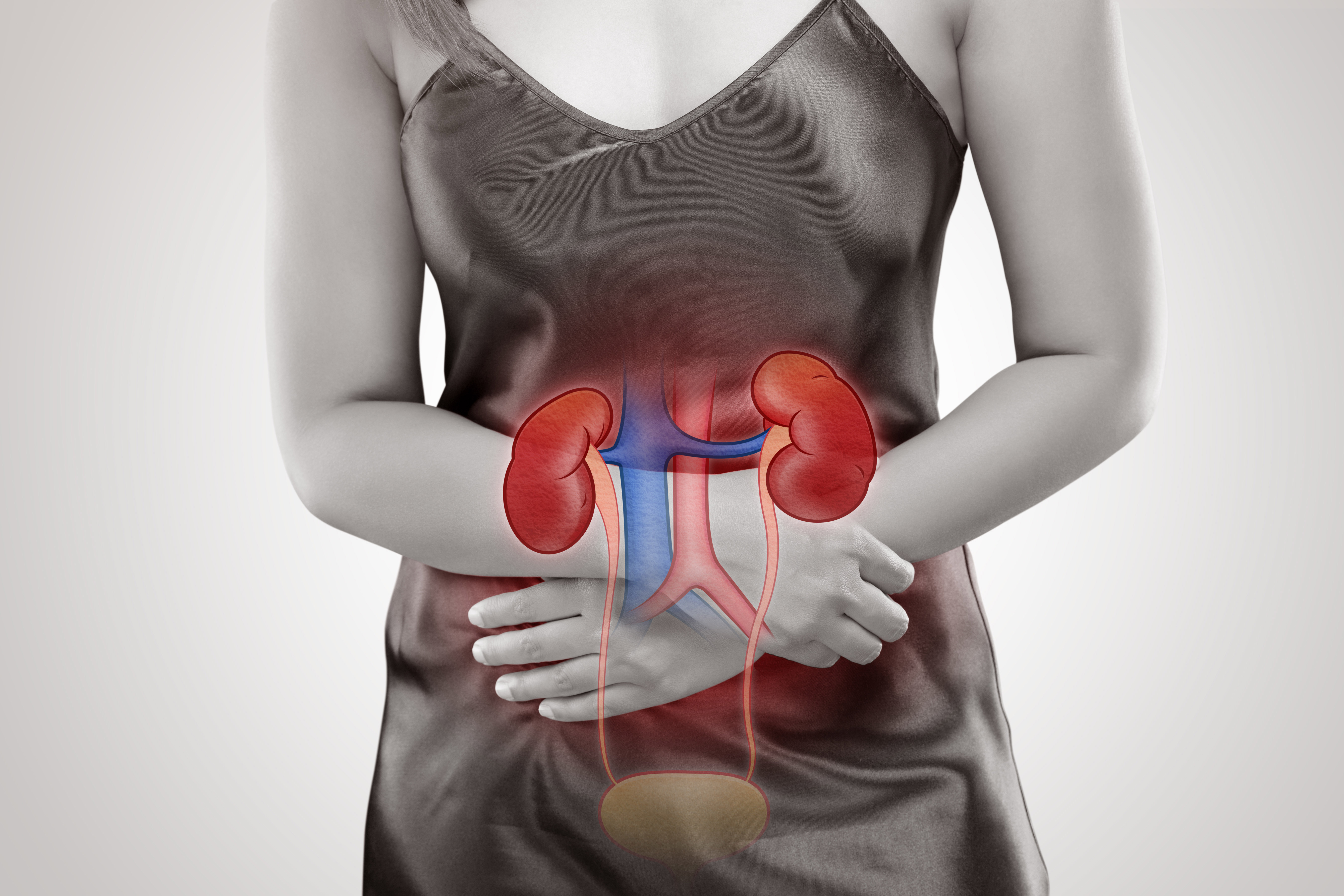
Pheochromocytomas are rare tumours of the adrenal glands. These glands are located right above the kidneys and make hormones, including stress hormones called epinephrines and norepinephrines.
Pheochromocytomas increase the production of these hormones, leading to hypertension (high blood pressure) and symptoms, such as headaches, irritability, sweating, rapid heart rate, nausea, vomiting, weight loss, weakness, chest pain, or anxiety. When this type of tumour occurs outside the adrenal gland, it is called a paraganglioma.